Adsorption of Solid¶
The strong adsorption forces on carbon nanotubes can also be used for binding other solid molecules. Similar to active carbon, the adsorptive properties can be used for the treatment of water contamination [241]. The most prominent usage, however, is the non-covalently functionalization of the surface, which enhances the solubility of individualized carbon nanotubes while mostly preserving their excitonic emission properties at room temperature.
Non-Covalent Functionalisation¶
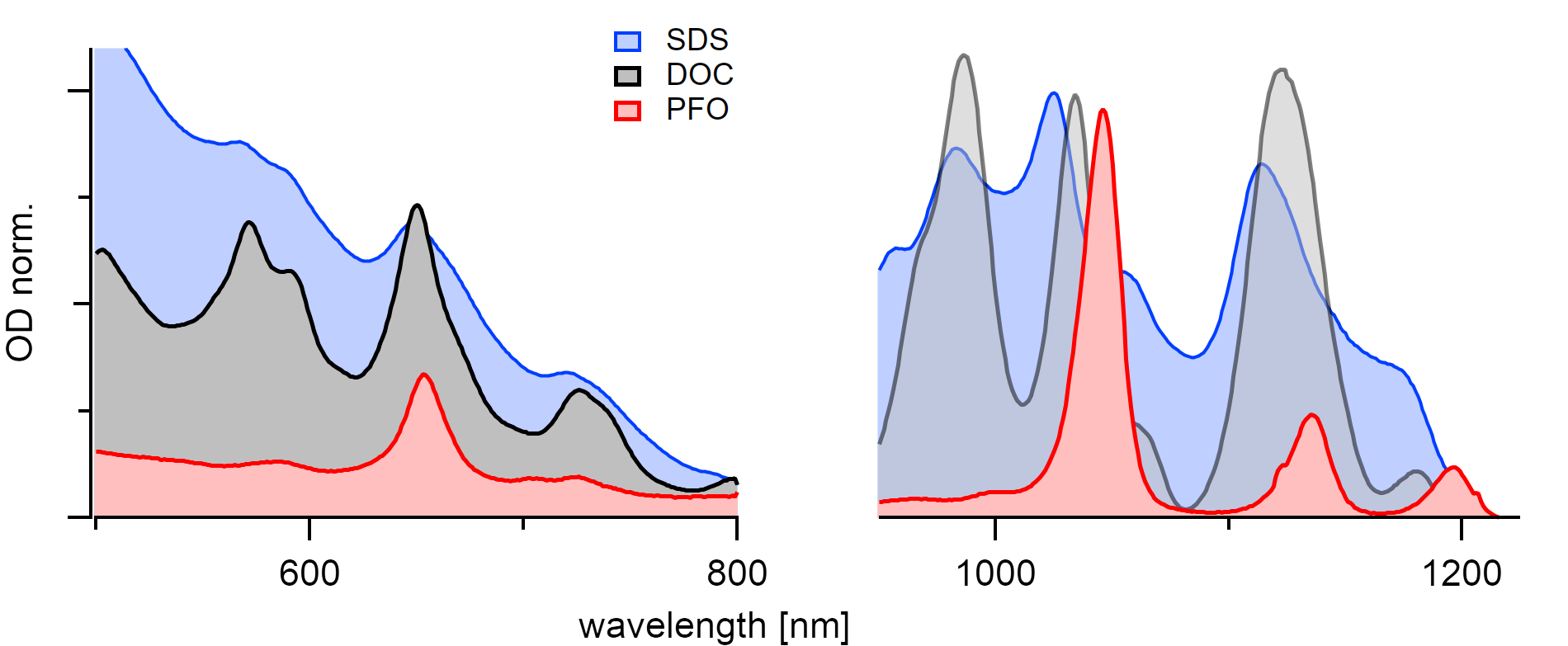
PFO selectively binds to the (7,5) type while DOC almost equally suspends every chirality. The rise in absorption background comes from the dispersion of graphene flakes and metallic nanotubes.¶
The first successful individualization of carbon nanotubes was achieved using sodium dodecyl sulfate (SDS) [242] in D₂O which encapsulates the nanotubes in micelles. Other amphiphilic molecules with water solubility like sodium dodecylbenzene sulfonate (SDBS) [243] followed. Both of the aforementioned sodium salts are in constant equilibrium with the surroundings leading to continuous ad- and desorption of the surfactants. Strong binding forces are crucial for creating kinetically stable suspensions [244] that preserves their emission properties on long time scales. Nowadays, thermodynamically more stable surfactants are employed to the advantage of better emission properties. In H₂O dispersions, cholic acid derivatives [101] or single-stranded DNA (ssDNA) are used. Furthermore, in organic solvents thiophene based polymers [245] like poly(3-hexylthiophene) (P3HT), fluorene based polymers [6][246][247] like poly(9,9‐dioctylfluorenyl‐2,7‐diyl) (PFO), thiophene-thiazol copolymers [248] like poly[2,6-(4,4-bis-(2- dodecyl)-4H-cyclopenta[2,1-b;3,4b’]dithiophene)-alt-4,7(2,1,3- benzothiadiazole)] (P12CPDTBT), and carbazole polymers [249] are used in combination with various other block co-polymers.
If the emission properties from nanotubes wrapped with different surfactants are compared in the same solvent, it is found that the type of surfactant plays a significant role for the excitonic energy as it also influences the dielectric environment. The transfer from DOC to SDBS, for example, causes a shift of the excitonic emission by 8nm [138], and SDS shows a strong blue-shift [243]. A time-dependent measurement of the photoluminescence during dilution shows that the desorption barrier of a polymer from the carbon nanotube surface is by far higher in organic solvents than micellular systems like ssDNA in water.[250] Such observations emphasize the higher stability of suspensions generated with the use of polymers compared to micellular systems. Upon these polymers, polyfluorenes are most frequently applied for the isolation and chirality-specific dispersion of individualized SWNTs in organic solvents [6], which is why in the following I want to investigate this system in more detail.
Adsorption of Polymer¶
The interaction of polymers with SWNT plays a key role in providing the desired selectivity. The mechanism for the adsorption of polymers, however, is under ongoing discussion.[7] In this section, the different binding processes are summarized to understand the interaction of polymers on nanotube surfaces and introduce the experiment design that is used for this study to help in clarifying the ongoing discussion.
P3HT is known to bind more strongly to SWNTs than PFO but has less selectivity which is why a thiophene based polymer can exchange the fluorene polymer if both are available in the solution.[251] Such behavior is typically explained by the rigidity of the backbone of PFO which controls the covalent binding strength of the π-stacking.[252] A flexible backbone gives stronger binding enthalpies and thus more stable suspensions. Also, for their selectivity, the rigidity of the polymer backbone plays a significant role. PFO has a more rigid backbone giving looser π-stacking, but this very rigidity leads to selective interaction with the (7,5) type while its more flexible block copolymer with bipyridine (PFO-byp) binds better to the (6,5) type but is less selective. It can be shown that the roll-up angle of an SWNT chirality influences the binding strength [247] of different co-polymers and thus alters the selectivity to certain chiralities. This roll-up angle dependence on the dispersion efficiency has been shown for various polymer combinations [249] which underlines the importance of both, π-stacking, and wrapping efficiency effects.
The rigidity and structure of the polymer backbone cause selectivity differences between PFO-bpy and PFO: PFO-bpy preferentially selects the (6,5) type, PFO, on the other hand, is suitable for selecting the (7,5) type.¶
Metallic nanotubes are responsible for the exponentially increasing strong absorption background [253] that is visible for SDS and DOC dispersed samples between 900-450nm. Polymers generally show little to no such background absorption. The high polarizability of metallic nanotubes is thought to lead to the effective removal of bundles [210] in organic solvents during centrifugation. Therefore, for measuring the selectivity of polymers, the solvent effects usually have to be differentiated from the pristine polymer effects that depend on the binding energies during π-stacking.[7] Brunecker et al. [250] also showed that for polymers, the de-sorption is neglectable. Consequently, ad-sorption measurements have to be performed on a clean surface because otherwise, the two competing processes are indistinguishable.
The dispersion efficiency for all polymers also strongly depends on the length and functionalization of the side chains.[245][254][244] SWNT that were processed with polymers that have longer side chains also show stronger absorption after sonication.[206] As the side chain length plays little to no role for the intrinsic optical absorption of the polymer [254], charge transfer can be excluded, and it is expected that the side chain length only increases solubility. The finding is affirmed by TDDFT calculations [255] and allows us to suggest that polymers adsorb with the aromatic backbone to the SWNT surface. One side-chain wrapping the nanotube surface and one side chain stands out to the solvent. Such solubility advantage effect of the dispersive medium is also found at high coverages of ssDNA [250] where the majority of the oligomers stands out to the solvent and is not directly attached to the nanotube surface. In polymer suspensions, two decay rates of the polymer fluorescence are observed [256], which can be explained with the difference of fluorescence between partly adsorbed polymer where some polymer trains are standing out to the solvent.
As outlined here, understanding the mechanism of non-covalent adsorptions on the nanotube surface is utterly important for the study of their emission properties. Single-molecule investigations were carried out using suspended carbon nanotubes to focus on this mechanism in more detail.
Single Molecules¶
A stable system that has gained considerable interest is the dispersion of carbon nanotubes with poly[(9,9-dioctylfluorenyl-2,7-diyl)-alt-co-(6,6’-{2,2’-bipyridine})] (PFO-bpy) in toluene.[247]. Investigations on the adsorption of to the (6,5) type show that the polymer wraps around the cylindrical nanotube at an angle of 14(2)° to the longitudinal axis.[257] Although the dispersion efficiency is low, these systems show more narrow line widths [218] than DOC wrapped nanotubes, and better quantum efficiencies than most other polymers.[213]
As outlined before, various effects during this adsorption process have to be considered, which is the reason for divergent interpretations and are mostly hard to distinguish. Most experiments in solution suffer from these overlapping phenomena. In this experiment, toluene-suspended SWNT are used as a defined starting point for studying polymer adsorption. The nanotubes are fixated on the substrate which hinders the nanotubes from precipitation and diffusion while maintaining the solvent environment during the investigation. It also allows to separate solvent and bundling effects from the usually concomitant polymer stacking and wrapping effects to investigate the undisturbed adsorption mechanism. Due to the selectivity for PFO-Bpy on smaller-diameter nanotubes, the experiment was performed with cooled silicon detectors to detect the emission between 830-1050nm. This allows investigating predominantly nanotubes of diameters between 0.7-0.8nm with a good quantum efficiency of the detectors to lower the integration times to 15-60s in solution with typical observation times of 5h. The high single spectrum acquisition time is owed to the generally lowered nanotube emission in solvents. For all SWNT with detectable diameters, PFO-Bpy is known to show selective dispersion. This allows investigating the rather low amount of nanotubes that survived the transfer to toluene while preserving their excellent emission properties.
The fluorescence of toluene-suspended carbon brightens after the addition of PFO-bpy. Some nanotubes even reach 75% of their initial emission in air.¶
The sample is placed into a quartz cuvette and wetted with an excess of 6mℓ toluene. After staying for at least 24h in the closed cuvette, 50-100µℓ of a 1.5nM polymer solution with an average polymer weight of 33kDa (60 monomers trains per molecule) is introduced. The sample is located at the bottom of the cuvette, and the wafer hinders the polymer from free diffusion to the area of observation. Nevertheless, the polymer is expected to reach the sample within a few minutes. After 24h of exposure to PFO-bpy, the emission intensity is around twice as high as in pristine toluene. This is most probably explained by the polymer leading to the desorption of toluene and preventing further adsorptions.
The excitonic emission in toluene with adsorbed PFO-Bpy is shifted to the red compared to the emission in pure toluene. The graph shows the unweighted spectral autocorrelation of individual measurements.¶
The polymer wrapping increases the dielectric environment. This increase leads to a red-shift of the excitonic emission signal. Energy shift and emission intensity are used as a probe for polymer adsorption during the time-dependent observation on single nanotubes. For this measurement, the nanotubes are first observed for 2h until emission stability at low excitation energies (5-30µW/3µm²). The adsorption of the polymer is observed in an excited thermodynamic equilibrium caused by the laser heat. This elevated state allows observing the ad- and desorption process on a single molecule basis.
Irreversible stepwise intensity decrease during the adsorption of PFO-bpy to a nanotube of the (6,4) type.¶
When observed over long time-scales, the emission intensity of the carbon nanotubes in solvents depleaches step-wise. Such depletion is probably caused by the generation of defects through reaction with oxygen which is known to generate irreversible steps.[160]
The time evolution of the energy is usually analyzed with the Allan deviation [258][259] to minimize the white noise in the emission spectra.[203] For nanotubes in toluene at room temperature with rather high integration times of 30s per spectrum, the energy fluctuations are minimal and are not expected to achieve a higher resolution with different statistics.
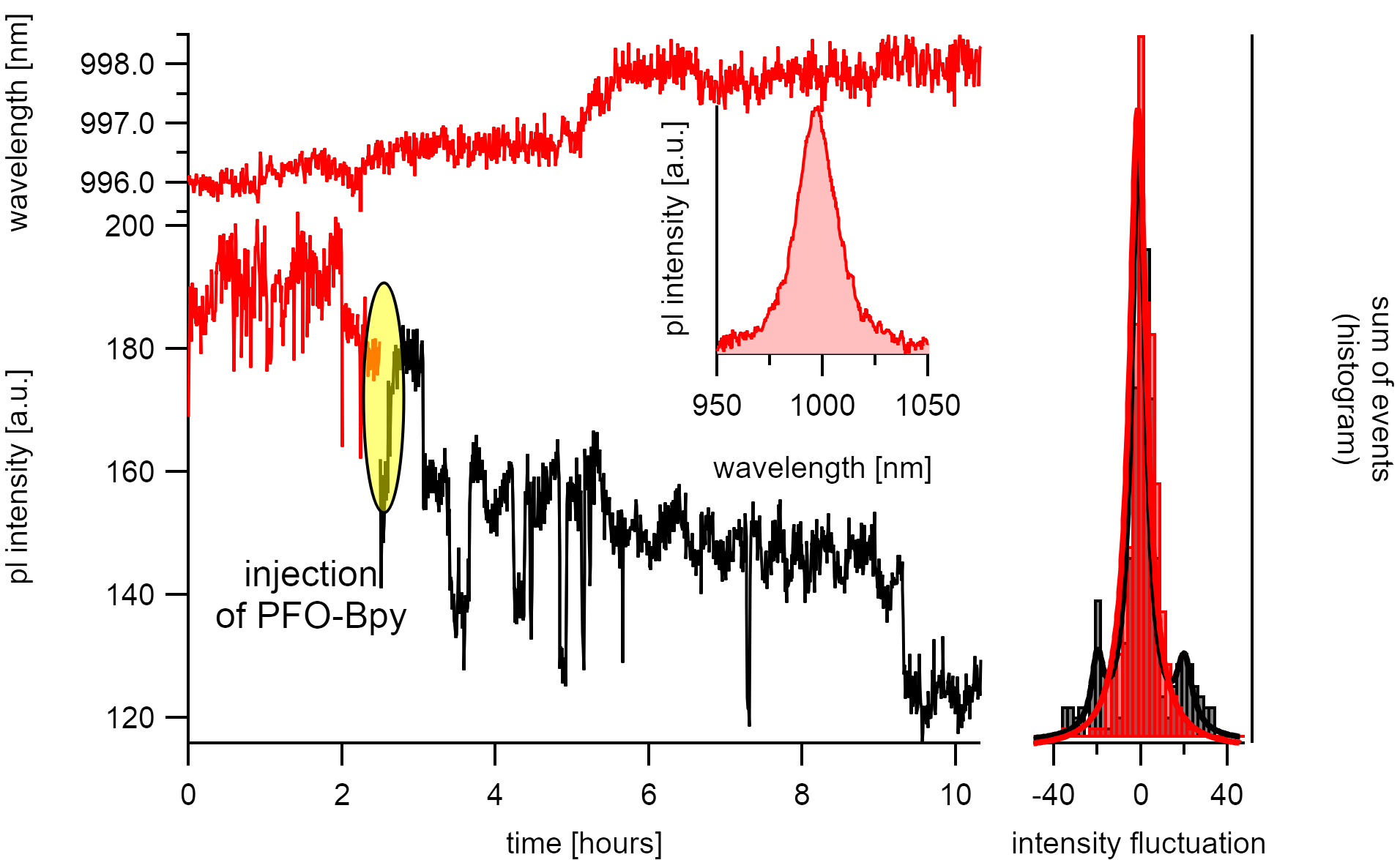
The figure shows the time-dependent adsorption of PFO-bpy on the (6,5) type. Adsorption is identified by an increase in the emission intensity and a red-shift of the emission wavelength.¶
There are also reversible steps visible that are caused by diffusion quenching within a mean segment size of Λ = 20/180 · 2µm = 220(20)nm. Such segment sizes are more than double the size as expected from the mean polymer train lengths. Unfortunately, high exciton diffusion lengths are expected for this system as proven with a simulation during image analysis. With such high diffusion lengths, the observation of polymer adsorption is limited. Nevertheless, the energy clearly shows a distinct shift to the red after the intensity fluctuations stop and the observed steps are partly reversibile. This allows assuming that we indeed observed polymer adsorption.
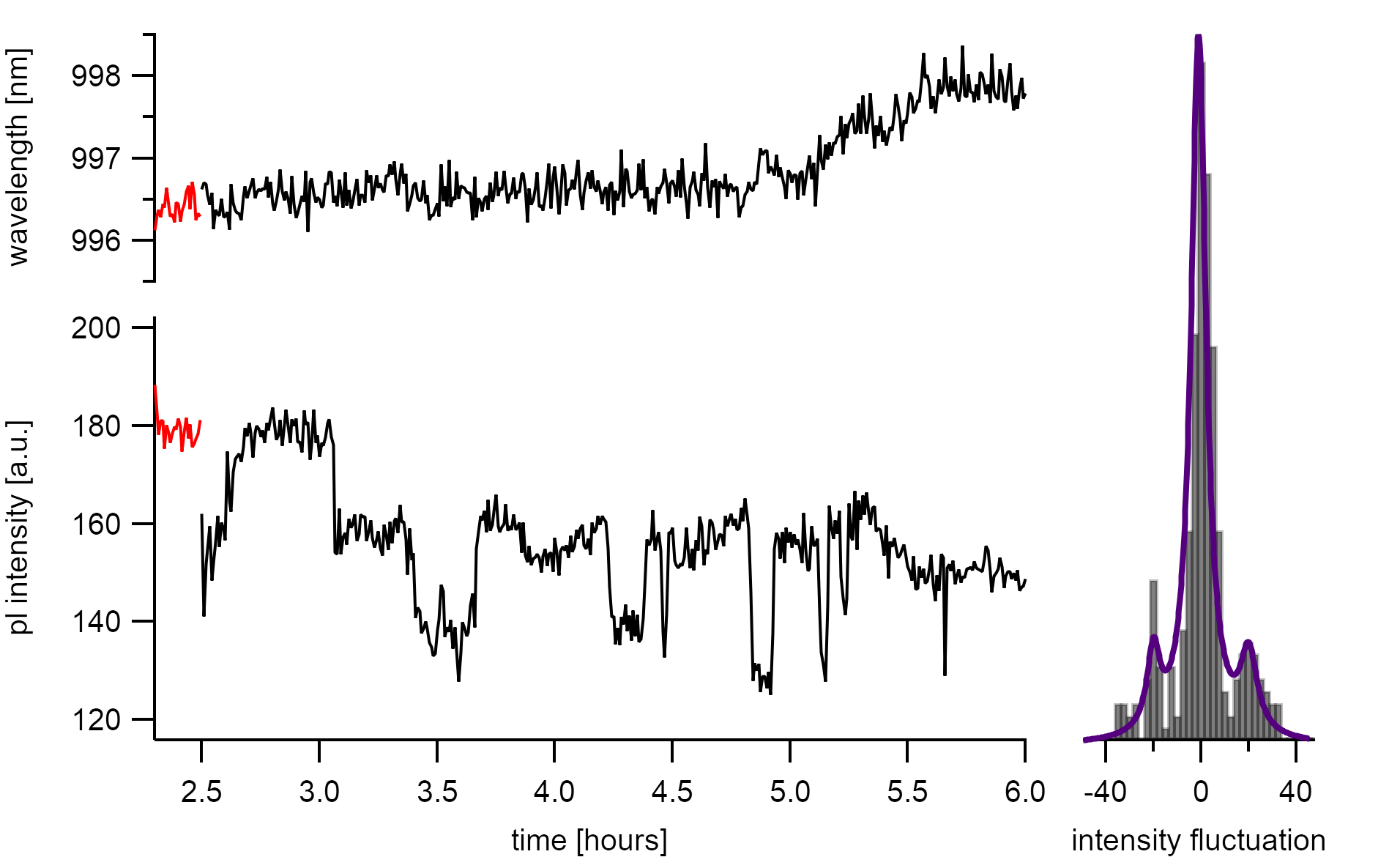
The intensity decreases stepwise. The irreversible increase in intensity is followed by a shift of the emission wavelength to the red.¶
The vague insights do not allow to make assumptions on the exact binding mechanism. A possible explaination of the observed signal could be that the the octyl groups form an initial binding while the rearrangement of the π-stacking from the polymer backbone to the exact angle around the nanotube axis takes longer. As toluene gets displaced by octyl, the energy shifts to the red.
Conclusion¶
The adsorption of individual polymer molecules on suspended nanotubes is a tedious and complicated measurement. Nevertheless, we presented initial results of the individual adsorption of Poly(9,9-di-n-octylfluorenyl-2,7-diyl)bipyridine (PFO-bpy) on the surface of suspended SWNTs in toluene using single molecule fluorescence microscopy and spectroscopy with single molecule observations over more than 6h. Changes in the intensity and central wavelength of the excitonic emission was used as a probe for surface effects.
It could be shown that the polymer only minimally changes the dielectric environment of the system and causes a red-shift compared to a system in toluene. The segment sizes of the involved polymer trains were below the resolvable limit defined by the exciton diffusion length. Such long polymer trains take rather long time for the irreversible adsorption. A cooperative binding mechanism is evident, although the exact mechanism leaves room for interpretation.